Limiting reactant pre lab answers hold the key to unlocking the mysteries of chemical reactions. Understanding this concept is paramount for predicting reaction outcomes, optimizing processes, and minimizing waste. This guide delves into the intricacies of identifying and applying the limiting reactant concept, empowering you to master the art of stoichiometry.
The journey begins with a comprehensive definition of the limiting reactant and an exploration of its pivotal role in determining the extent of a chemical reaction. Step-by-step methods for identifying the limiting reactant are meticulously laid out, accompanied by illustrative examples and clear explanations.
Introduction

In a chemical reaction, the limiting reactant is the reactant that is consumed first, thereby limiting the amount of product that can be formed. Identifying the limiting reactant is crucial for predicting the maximum yield of the reaction and optimizing its efficiency.
Methods for Identifying the Limiting Reactant
To identify the limiting reactant, follow these steps:
- Balance the chemical equation to determine the stoichiometric ratio of the reactants.
- Convert the given quantities of each reactant to moles using their respective molar masses.
- Divide the number of moles of each reactant by its stoichiometric coefficient in the balanced equation.
- The reactant with the smallest value obtained in step 3 is the limiting reactant.
Example:
Consider the reaction: 2A + 3B → C
If we have 10 moles of A and 15 moles of B, the limiting reactant is determined as follows:
- Moles of A = 10 mol
- Moles of B = 15 mol
- Moles of A/stoichiometric coefficient (A) = 10 mol/2 = 5 mol
- Moles of B/stoichiometric coefficient (B) = 15 mol/3 = 5 mol
Since both reactants have the same value (5 mol), they are consumed simultaneously, and neither is limiting.
Factors Affecting the Limiting Reactant
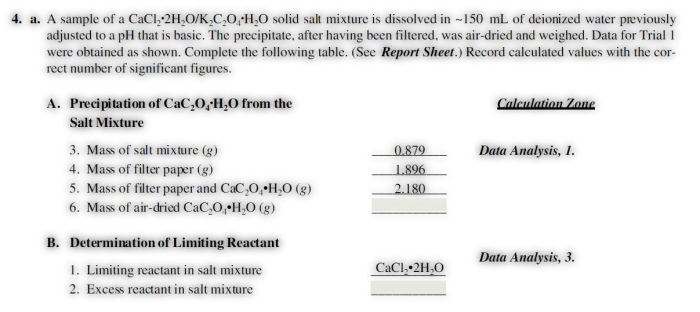
The identity of the limiting reactant can be affected by several factors, including:
- Initial quantities of reactants:The limiting reactant is the one present in the smallest initial amount relative to its stoichiometric ratio.
- Stoichiometric coefficients:The coefficients in the balanced equation determine the relative amounts of reactants required for complete reaction. Larger coefficients indicate a greater demand for that reactant.
- Impurities:The presence of impurities can reduce the effective amount of a reactant, potentially altering the limiting reactant.
Applications of Limiting Reactant Concept: Limiting Reactant Pre Lab Answers

The limiting reactant concept has numerous applications in various fields, including:
- Chemistry:Predicting product yields, optimizing reaction conditions, and understanding reaction mechanisms.
- Industry:Determining optimal reactant ratios for chemical processes, minimizing waste, and maximizing efficiency.
- Environmental science:Assessing the limiting factors in environmental reactions, such as nutrient availability in ecosystems.
Common Queries
What is the significance of identifying the limiting reactant?
Identifying the limiting reactant is crucial because it determines the maximum amount of product that can be formed in a reaction. It allows chemists to optimize reactions, predict yields, and minimize waste.
How do I calculate the limiting reactant in a reaction?
To calculate the limiting reactant, compare the mole ratios of the reactants to their stoichiometric coefficients. The reactant with the smallest mole ratio relative to its coefficient is the limiting reactant.
What factors can affect the identity of the limiting reactant?
Factors that can affect the identity of the limiting reactant include the initial amounts of reactants, the stoichiometry of the reaction, and the presence of side reactions.

