Describe the relative densities of the phases for most substances – In the realm of chemistry, understanding the relative densities of different phases is crucial for comprehending the behavior of substances. This guide delves into the concept of relative density, exploring the factors that influence it and its applications in various fields.
Relative density, a measure of a substance’s density relative to a reference substance, provides valuable insights into the packing of molecules and the overall density of a substance. Temperature, pressure, and composition are among the key factors that can significantly affect relative density.
Relative Densities of Different Phases
Relative density is a measure of the density of a substance relative to the density of a reference substance, typically water at 4 °C. It is a dimensionless quantity and is often expressed as a specific gravity. The relative density of a substance provides information about its mass and volume, and it can be used to identify and characterize substances, determine their purity, and predict their behavior under different conditions.The
relative density of a substance is affected by several factors, including temperature, pressure, and composition. Temperature and pressure can affect the packing of molecules and the overall density of the substance. For example, as the temperature of a substance increases, its relative density typically decreases due to the increased molecular motion and expansion of the substance.
Similarly, as the pressure on a substance increases, its relative density typically increases due to the compression of the substance.The composition of a substance can also affect its relative density. For example, the addition of impurities to a substance can decrease its relative density, while the removal of impurities can increase its relative density.The
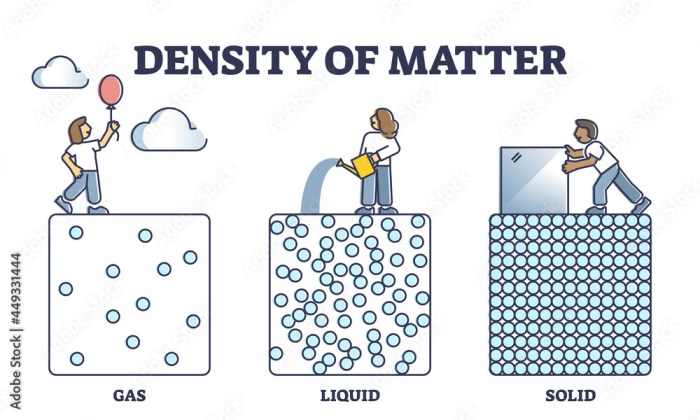
relative densities of different phases of a substance can vary significantly. Solids typically have the highest relative density, followed by liquids and then gases. This is because solids have a tightly packed molecular structure, while liquids have a less tightly packed molecular structure, and gases have a very loosely packed molecular structure.For
example, the relative density of water at room temperature is approximately 1.00 g/cm 3. The relative density of ice at 0 °C is approximately 0.92 g/cm 3, while the relative density of water vapor at room temperature is approximately 0.0006 g/cm 3.The
relative densities of different phases of a substance can be used to identify and characterize substances, determine their purity, and predict their behavior under different conditions. For example, the relative density of a substance can be used to determine whether it is a solid, liquid, or gas.
The relative density of a substance can also be used to determine the purity of a substance. For example, the presence of impurities in water can decrease its relative density, which can be used to determine the purity of the water.The
relative densities of different phases of a substance can also be used to predict their behavior under different conditions. For example, the relative density of a substance can be used to predict how it will float or sink in a liquid.
The relative density of a substance can also be used to predict how it will behave under pressure.
Helpful Answers: Describe The Relative Densities Of The Phases For Most Substances
What is relative density?
Relative density is a measure of a substance’s density relative to a reference substance, typically water for liquids and solids and air for gases.
Why is relative density important?
Relative density provides valuable insights into the packing of molecules and the overall density of a substance, which can influence its behavior and properties.
What factors can affect relative density?
Temperature, pressure, and composition are among the key factors that can significantly affect the relative density of a substance.